The Food and Drug Administration's outside panel of advisers will have a lot to consider this month, as multiple meetings on booster shots and COVID vaccines for kids are now on the schedule.
According to the agency, the Vaccines and Related Biological Products Advisory Committee is scheduled to meet three times in October to discuss whether to recommend emergency use authorization of Moderna and Johnson & Johnson's booster shots and vaccinations for children ages 5 to 11.
The booster shot meetings are slated for Oct. 14-15, where the panel will review booster data from both J&J and Moderna. It’s the first step in a review process that also includes sign-off from the leadership of both the FDA and the Centers for Disease Control and Prevention. If both agencies give the go-ahead, Americans could begin getting J&J and Moderna boosters later this month.
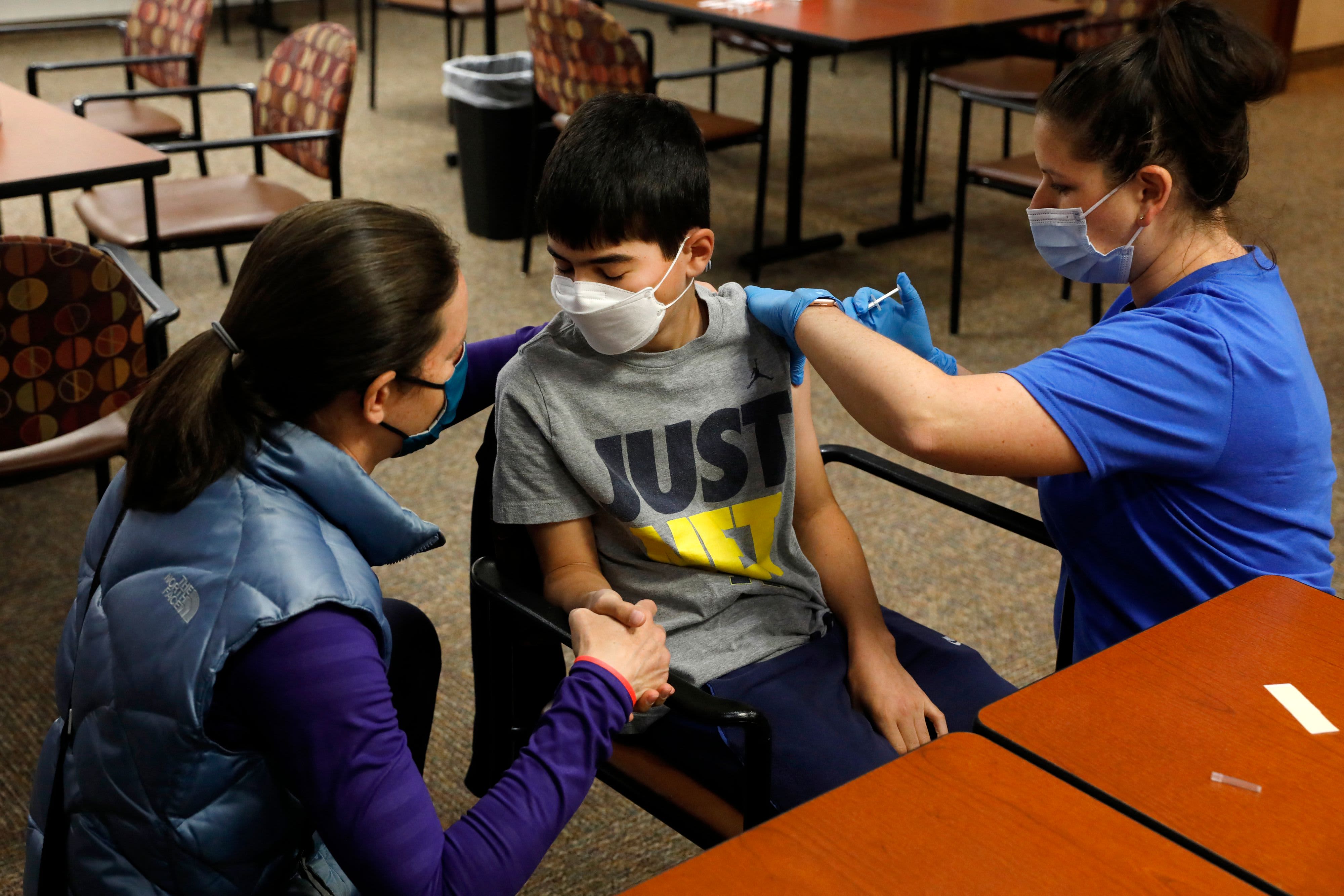
After that, the same panel will consider vaccinations for children under the age of 12. The group will consider whether emergency use authorization should be granted for Pfizer's COVID vaccine for children 5 to 11 years old.
Get Boston local news, weather forecasts, lifestyle and entertainment stories to your inbox. Sign up for NBC Boston’s newsletters.
Pfizer and its German partner BioNTech said Thursday they have made a formal request to the Food and Drug Administration to extend emergency use of its COVID-19 vaccine in children ages 5 to 11.
"We're committed to working with the FDA with the ultimate goal of helping protect children against this serious public health threat," Pfizer tweeted.
The announcement came one week after they companies submitted initial trial data to the Food and Drug Administration for use of its COVID-19 vaccine in younger children.
Last month, the companies announced topline results of trial data that found the vaccine to be safe, while prompting a "well tolerated" and "robust" antibody response among the younger, targeted group.
The two-dose vaccine is already authorized in teens aged 12 to 15 and fully approved for ages 16 and up. But with kids now back in school and the extra-contagious delta variant causing a huge jump in pediatric infections, many parents are anxiously awaiting vaccinations for their younger children.
Now the FDA will have to decide if that evidence is strong enough to expand use to youngsters. An independent expert panel will publicly debate the evidence on Oct. 26.
If the FDA authorizes emergency use of the kid-sized doses, there’s another hurdle before vaccinations in this age group can begin. Advisers to the Centers for Disease Control and Prevention will decide whether to recommend the shots for youngsters, and the CDC will make a final decision.
Meanwhile, Johnson & Johnson asked the FDA on Tuesday to allow extra shots of its COVID-19 vaccine as the U.S. government moves toward expanding its booster campaign to millions more vaccinated Americans.
The timing of the J&J filing was unusual given that the FDA had already scheduled its meeting on the company's data. Companies normally submit their requests well in advance of meeting announcements. A J&J executive said the company has been working with FDA on the review.
“Both J&J and FDA have a sense of urgency because it’s COVID and we want good data out there converted into action as soon as possible,” said Dr. Mathai Mammen, head of research for J&J's Janssen unit.
Bloomberg, citing people familiar with the matter, reported last week that the FDA was leaning toward authorizing half-dose booster shots for those who received Moderna's two-shot mRNA vaccine.
Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept. 1.
“We are pleased to initiate the submission process for our booster candidate at the 50 µg dose with the FDA. Our submission is supported by data generated with the 50 µg dose of our COVID-19 vaccine, which shows robust antibody responses against the Delta variant,” Stéphane Bancel, Moderna's CEO, said in a statement.
Moderna previously released data on breakthrough cases, saying it supports the push for wide use of COVID-19 vaccine booster shots.