Three Massachusetts doctors are on the U.S. Food and Drug Administration’s vaccine advisory panel that met Thursday to discuss whether or not the agency should approve Pfizer's COVID-19 vaccine for emergency use.
Drs. Cody Meissner from Tufts, Eric Rubin from Harvard T.H. Chan School of Public Health, and Ofer Levy from Boston Children's Hospital and the rest of the FDA advisory committee participated in a virtual meeting Thursday. The panel recommended that Pfizer’s coronavirus vaccine should be approved for emergency use authorization.
Get Boston local news, weather forecasts, lifestyle and entertainment stories to your inbox. Sign up for NBC Boston’s newsletters.
While Levy, who is director of the Precision Vaccines Program at Boston Children’s, said ahead of the vote he was optimistic.
“I think overall, the data look very positive,” Levy said. “I’ve not announced how I’m going to vote. I’m obviously enthusiastic about the data but we need to let the process take its course.”
WATCH LIVE: FDA Panel Meets to Consider Pfizer's COVID Vaccine
During the day-long meeting the panel looked at all the data and heard from experts and the public.
“I can tell from the briefing documents that no corners have been cut here,” Levy said. “It’s a very complete report on the safety features and I said largely what was seen was mild and moderate side effects.”
More Coverage on Coronavirus in Mass.
This is a two-dose vaccine and Levy said safety will continue to be monitored.
There are some outstanding questions about the vaccine, such as how long it lasts and what the effects are on children and pregnant women, but Levy said he was encouraged by what he’s seen so far.
“Developing something that safe and that effective in this amount of time is unprecedented and potentially a very important step forward in addressing this pandemic,” said Levy. “It looks like there might start to be some light at the end of the tunnel here.”
The FDA could issue an emergency use authorization by the end of Thursday. If that happens, Pfizer’s vaccine could then be distributed within 24 hours.
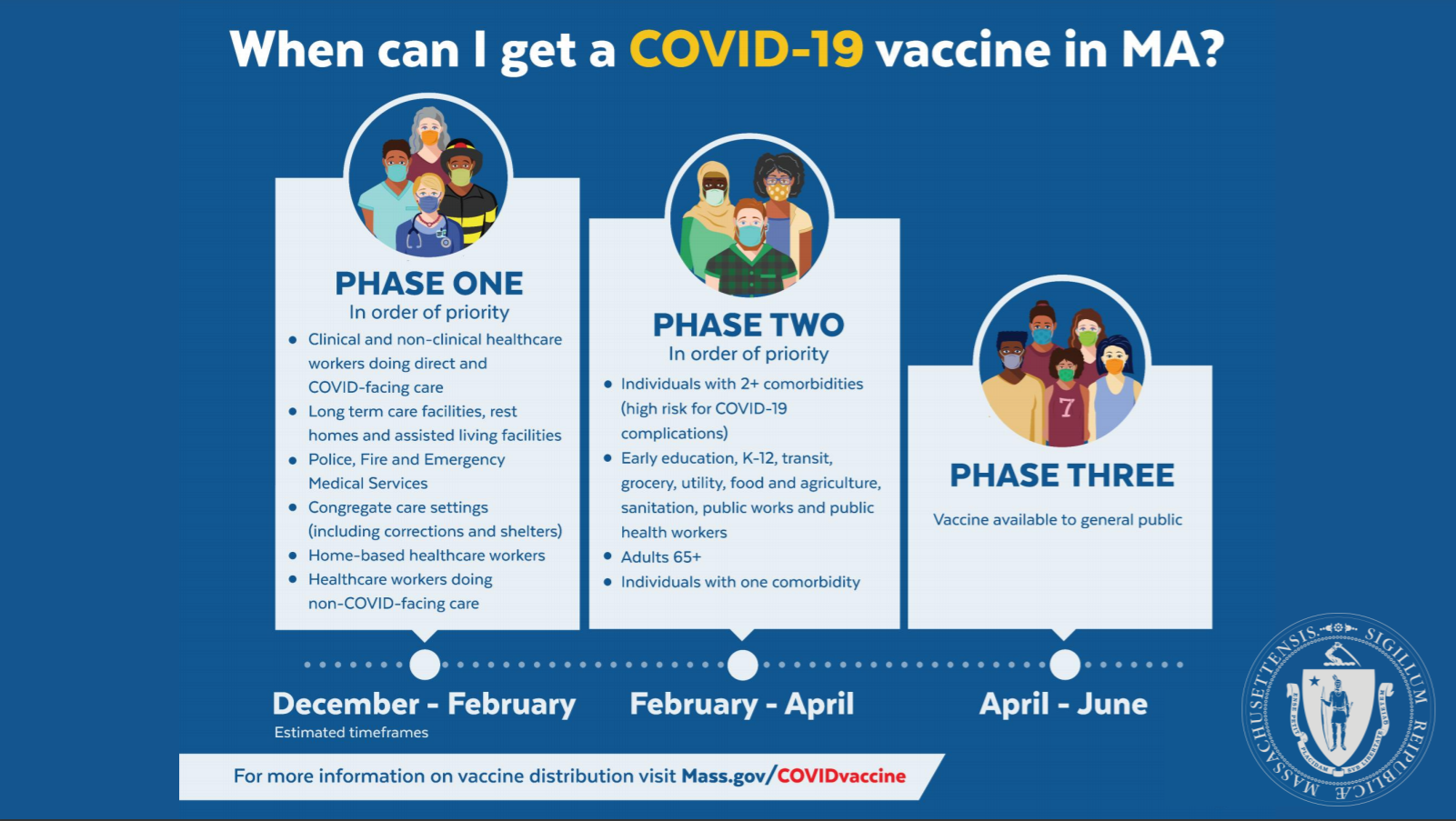
On Wednesday, Gov. Charlie Baker detailed the plan to distribute the coronavirus vaccine in Massachusetts once it is approved by the FDA.
It’ll occur in three phases and is expected to start before the end of the month, Baker said. The first group will include healthcare workers, people in long-term care, and first responders, as well as inmates at correctional facilities.